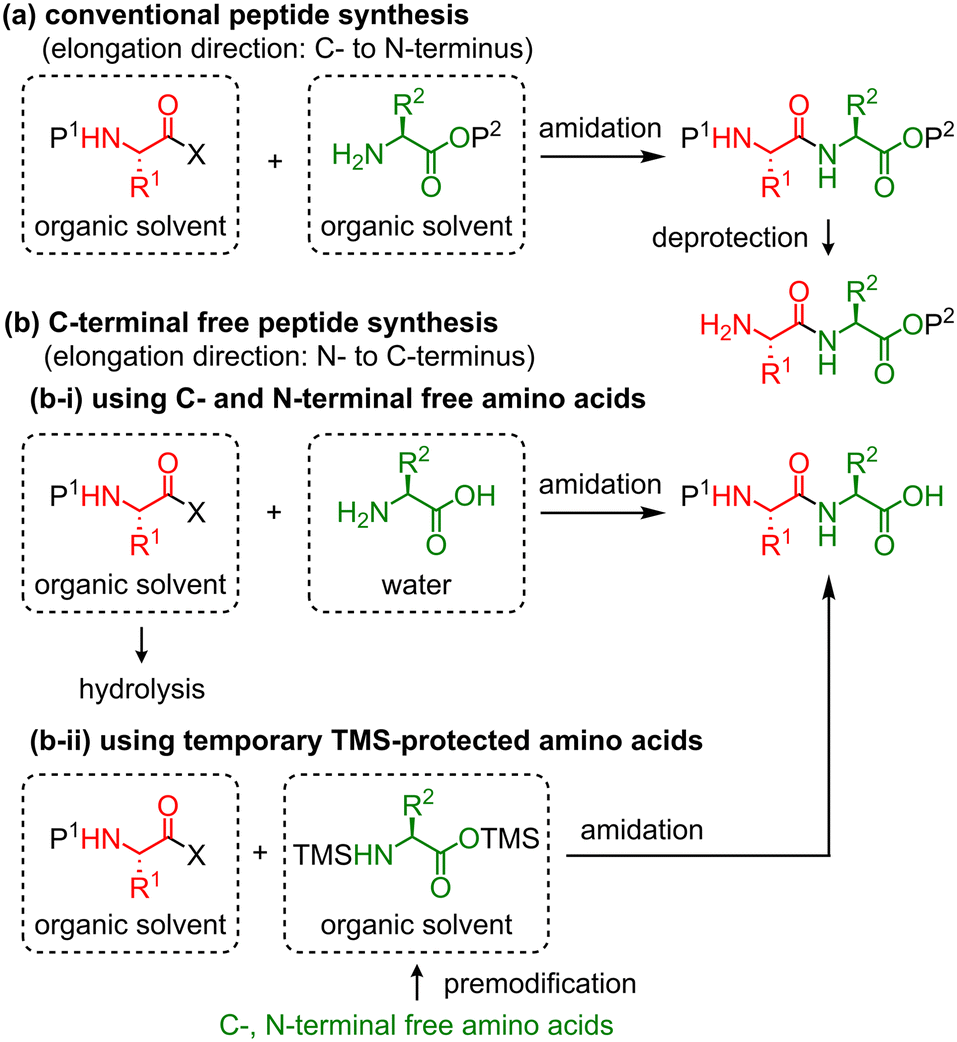
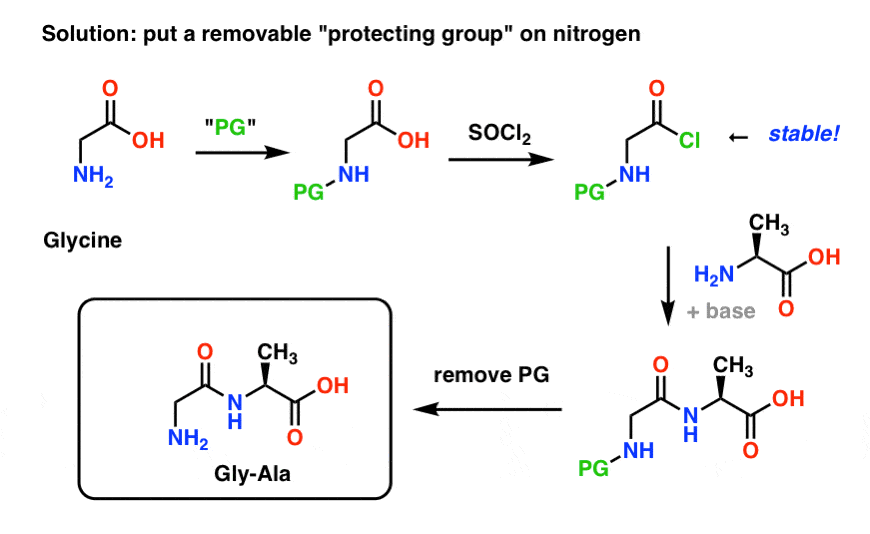
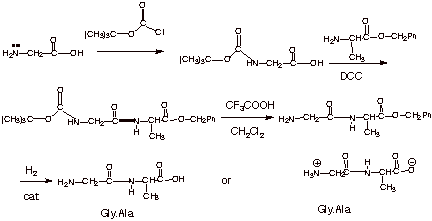
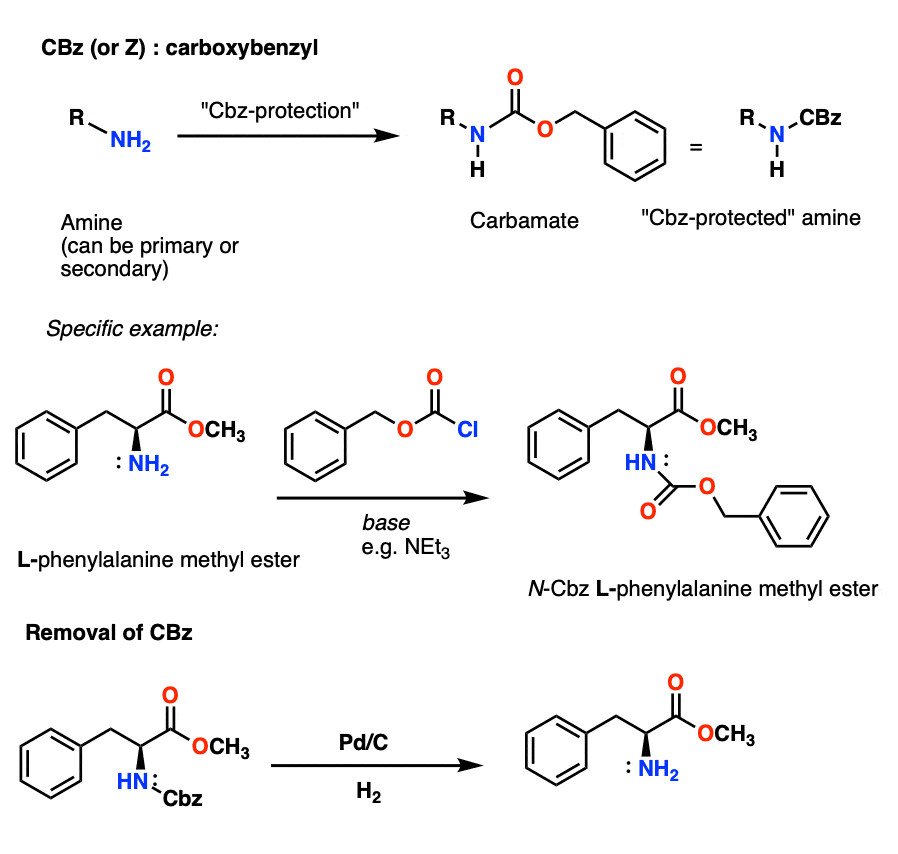
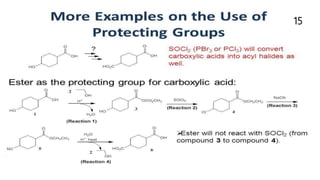
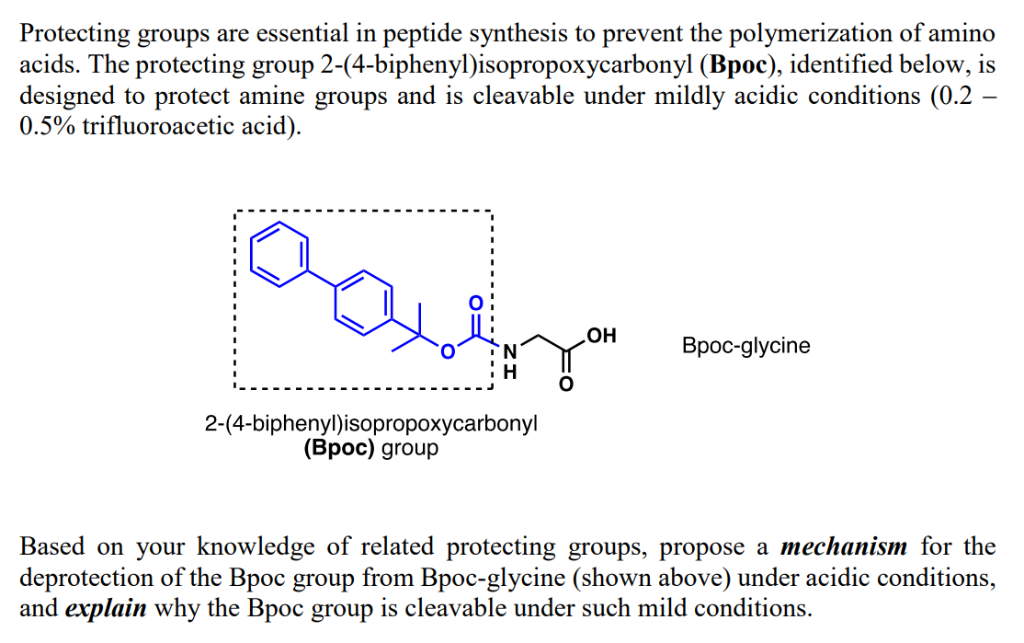
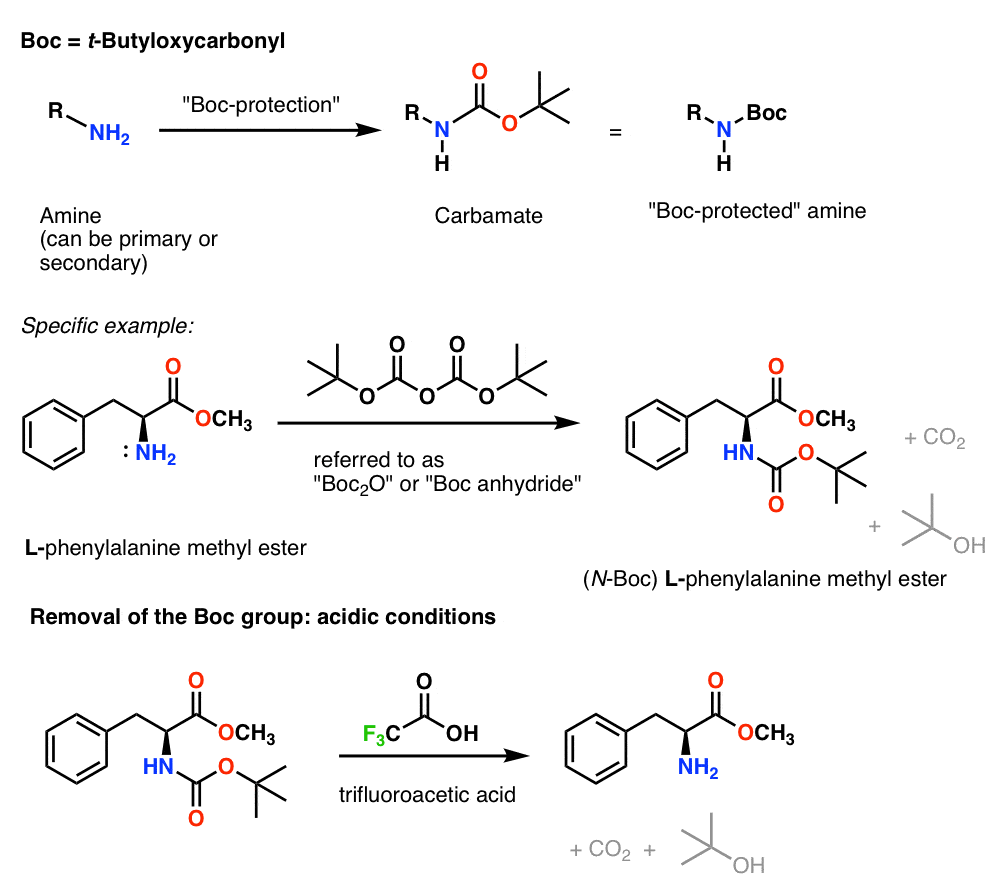
Chemical structure and short names of TFA-labile protecting groups used... | Download Scientific Diagram

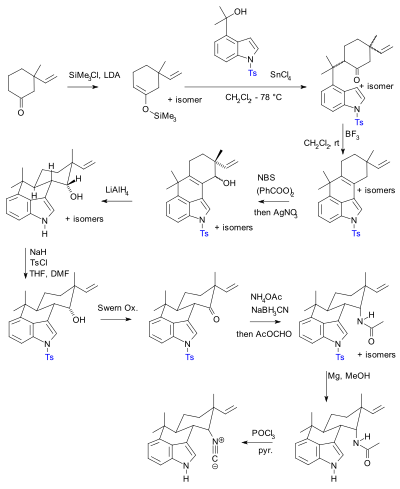
Utilization of Fukuyama's sulfonamide protecting group for the synthesis of N-substituted α-amino acids and derivatives - ScienceDirect

Orthogonal protecting groups for Nα‐amino and C‐terminal carboxyl functions in solid‐phase peptide synthesis - Albericio - 2000 - Peptide Science - Wiley Online Library

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications
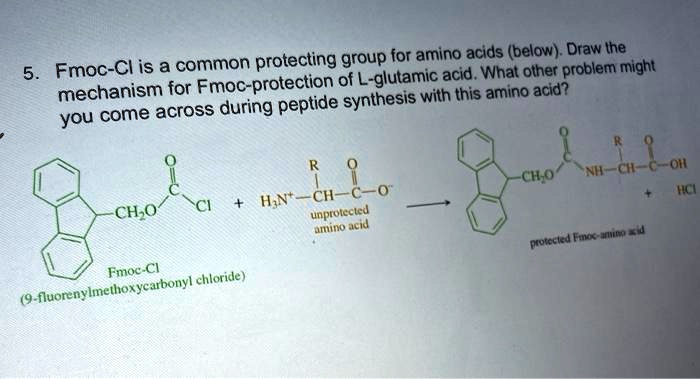
SOLVED: Fmoc-Cl is a common protecting group for amino acids. What other problem might arise with this amino acid during peptide synthesis?

Molecules | Free Full-Text | Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green Calcium(II) Iodide as a Protective Agent