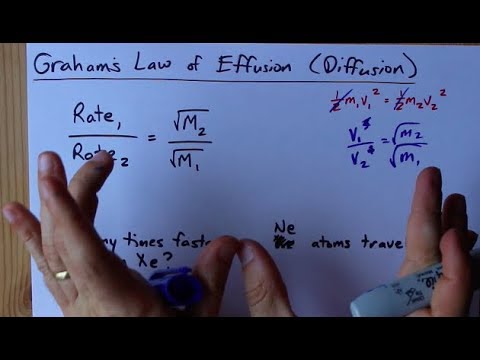
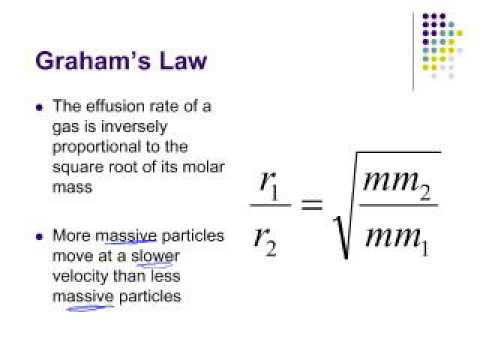
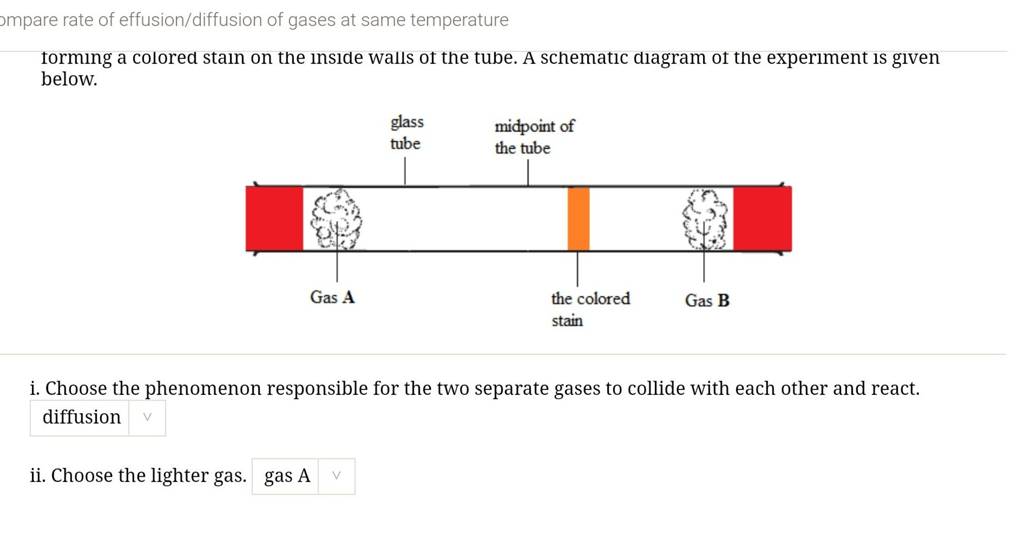
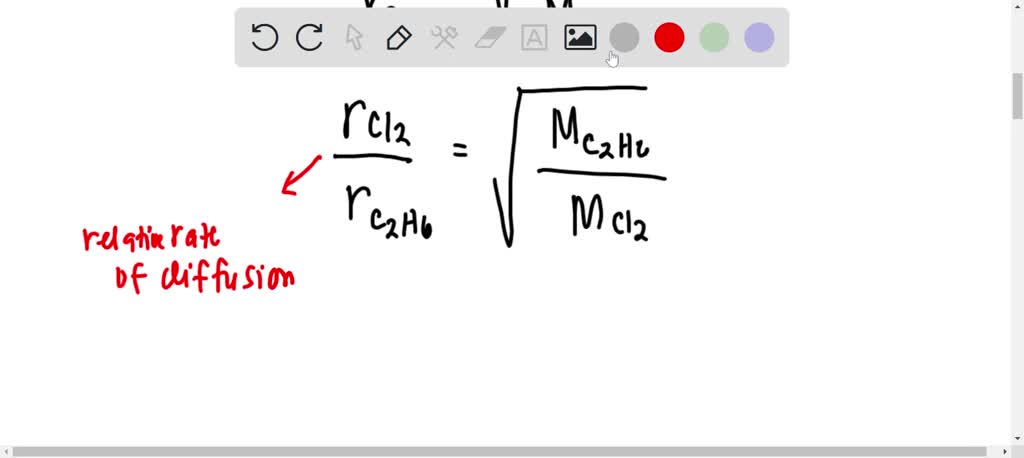
The rate of diffusion of two gases X and Y is in the ratio of 1:5 and that of Y and Z in the ratio of I: 6. The ratioof the rate
The ratio of rates of diffusion of 2 gases A & B is 1:5.If the relative molecular mass of A is 16,what is the relative molecular mass of B? - Quora
63.Rate of diffusion of gas X is twice that of gas Y if molecular mass of Y is 64 then the molecular mass of X will be
34. if the ratio of Rates of diffusion of two gases X and Y is 9 : 1 the ratio of their densities is

SOLVED: In this experiment, we will measure the diffusion rates of hydrogen chloride gas (HCl), ammonia gas (NH3), and ammonium gas (NH4) obtained from hydroxide and hydrochloric acid solutions. When these gases

What is the ratio of the rate of diffusion of helium gas to that of oxygen under identical condi... - YouTube

The rate of diffusion of a gas X is √(2) times that of Y . If the molecular weight of X is 16 , the molecular weight of Y is:

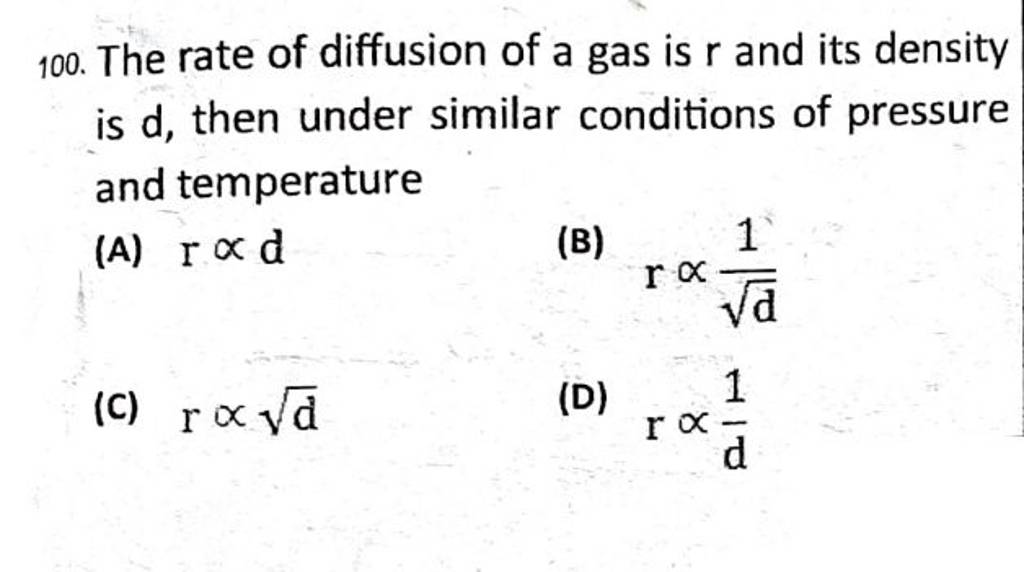

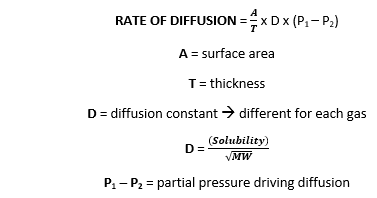
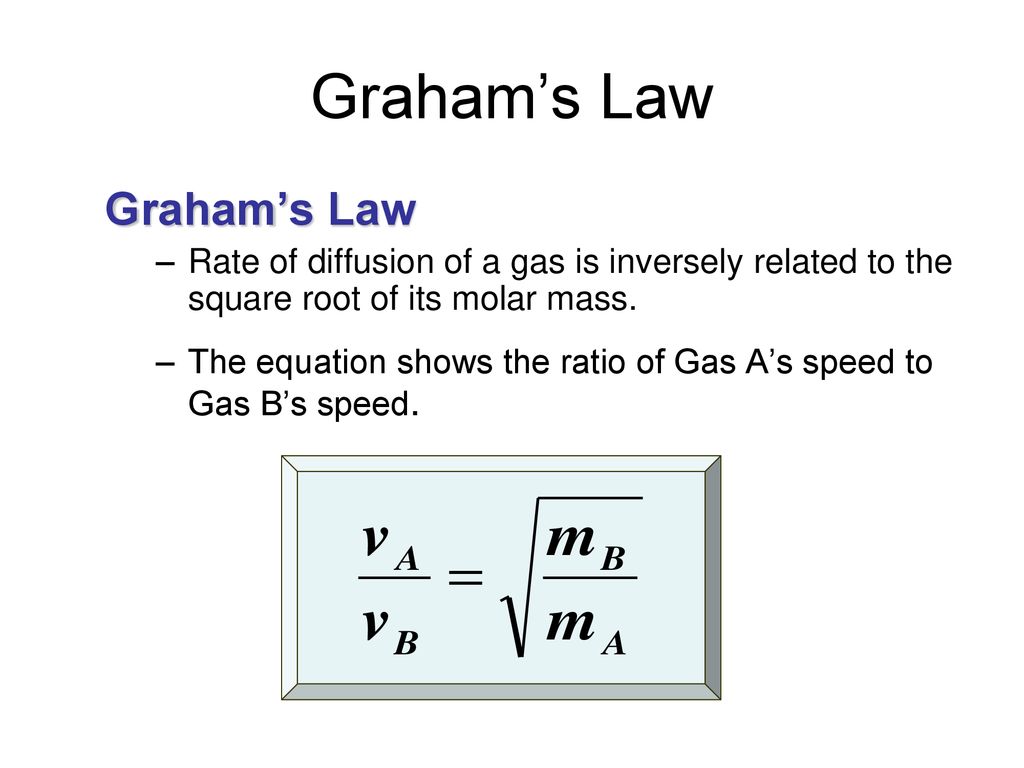




![Telugu Solution] Rate of diffusion of gases is not influenced by Telugu Solution] Rate of diffusion of gases is not influenced by](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/7393517.webp)